
Randomized study with a run-in dose-selection phase to assess the added value of
lenalidomide in combination with standard remission-induction chemotherapy and
post-remission treatment in patients aged 18-65 years with previously untreated acute
myeloid leukemia (AML) or high risk myelodysplasia (MDS) (IPSS-R risk score > 4.5)
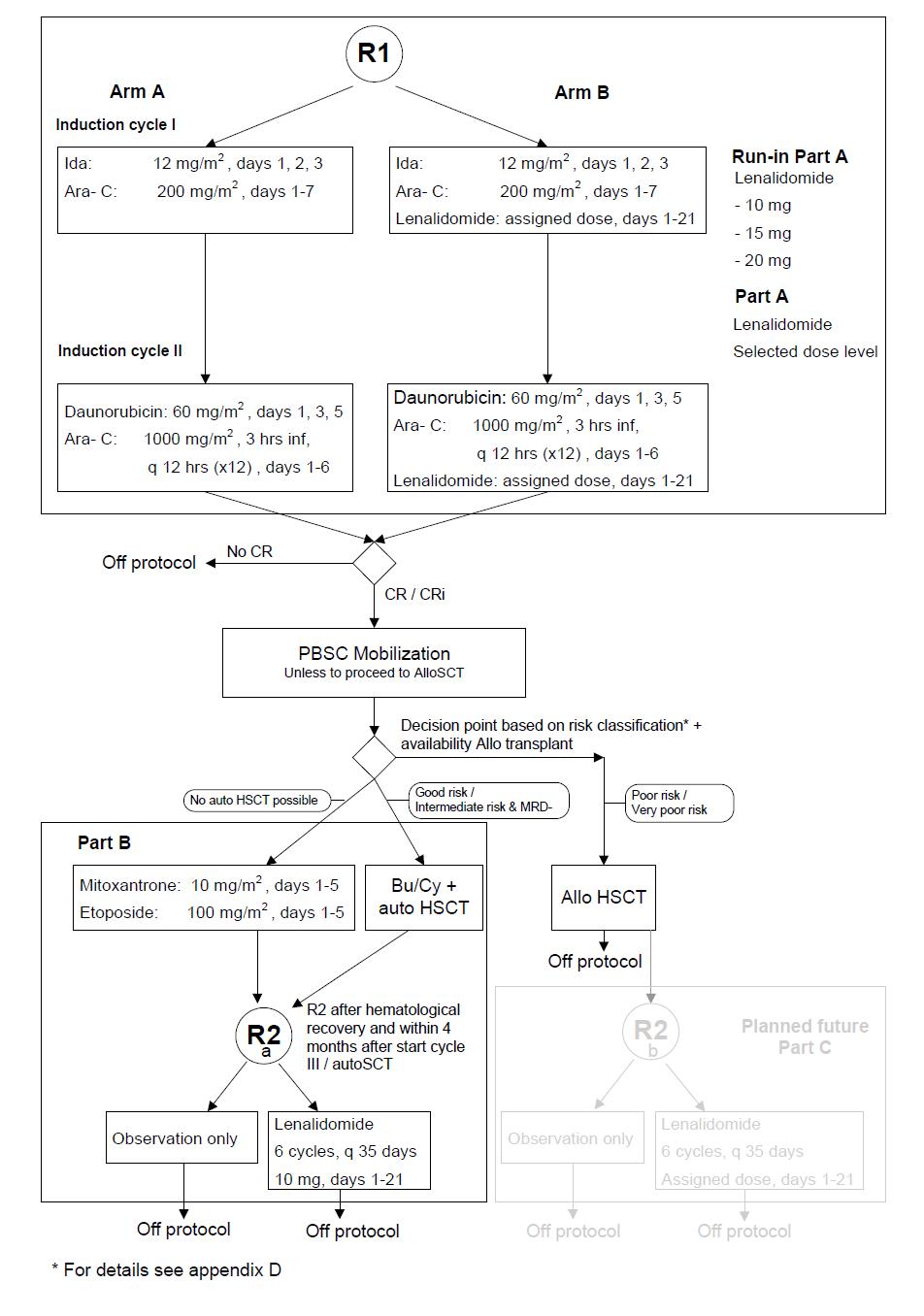
To select in a randomized approach the feasible dose level of lenalidomide when given orally at three variable dose levels (at 20 mg/day 1-21; 15 mg/day 1-21 or 10 mg/day 1-21) in combination with standard induction cycles I and II in patients with AML/ MDS with IPSS-R> 4.5.
To evaluate the effect of lenalidomide on EFS (Event Free Survival) at the selected feasible dose level when combined with remission induction chemotherapy cycles I and II in a randomized comparison to remission induction cycles I and II without addition of lenalidomide.
To evaluate the effect on the Cumulative incidence of relapse (CIR) of 6 cycles of maintenance therapy with lenalidomide treatment (10 mg/day for 21 days followed by 14 days rest) after post remission chemotherapy cycle III or autoHSCT versus observation only.
o To investigate the efficacy of lenalidomide in combination with remission induction chemotherapy cycles I and II (in comparison with the same treatment without lenalidomide) in all patients with regard to complete remission rate (CR/ CRi), DFS, CIR and OS
o To investigate the efficacy of lenalidomide in combination with remission induction chemotherapy cycles I and II in molecularly and cytogenetically distinguishable subsets with regard to complete remission rate (CR/CRi), DFS, CIR and OS
o To evaluate the treatment effects according to MRD measurements following therapy by standardized sampling of bone marrow/blood following remission induction treatment
o To determine the prognostic value of molecular markers and gene expression profiles of the leukemia cells assessed at diagnosis for both remission induction treatments
o To investigate the toxicities of lenalidomide in combination with remission induction chemotherapy cycles I and II
o To compare CIR after autoHSCT and cycle III according to molecular markers and MRD measurements
o To evaluate the effect of lenalidomide on the feasibility of collecting adequate autologous stem cell grafts and the probability of proceeding to autoHSCT
o To investigate the efficacy of lenalidomide with regard to DFS and OS measured from 2nd randomization
o To investigate post remission and post-transplant toxicities and need for transfusions when lenalidomide is applied after post remission chemotherapy/autoHSCT
o To evaluate the efficacy of lenalidomide as post-remission therapy to prevent relapse in all randomized patients, but also in relationship with the distinctive risk categories of AML (as based on cytogenetics and molecular genetics) and MRD estimates
o a diagnosis of AML and related precursor neoplasms according to WHO 2008 classification (excluding acute promyelocytic leukemia) including secondary AML (after an antecedent hematological disease (e.g. MDS) and therapy-related AML), or
o acute leukemia’s of ambiguous lineage according to WHO 2008 or
o diagnosis of refractory anemia with excess of blasts (MDS) and IPSS-R score > 4.5
o Serum creatinine ≤1.0 mg/dL (≤88.7 μmol/L); if serum creatinine >1.0 mg/dL (>88.7μmol/L), then the estimated glomerular filtration rate (GFR) must be >60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease equation where Predicted GFR (ml/min/1.73 m2) = 186 x (Serum Creatinine in mg/dL)-1.154 x (age in years)-0.203 x (0.742 if patient is female) x (1.212 if patient is black) NOTE: if serum creatinine is measured in umol/L, recalculate it in mg/dL according to the equation: 1 mg/dL = 88.7 umol/L) and use above mentioned formula.
o Serum bilirubin ≤2.5 x upper limit of normal (ULN)
o Aspartate transaminase (AST) ≤ 2.5 x ULN
o Alanine transaminase (ALT) ≤ 2.5 x ULN
o Alkaline phosphatase ≤ 2.5 x ULN
o basal and squamous cell carcinoma of the skin
o in situ carcinoma of the cervix
o Myocardial infarction within the last 6 months of study entry, or
o Reduced left ventricular function with an ejection fraction < 50% as measured by MUG scan or echocardiogram or
o Unstable angina, or
o Unstable cardiac arrhythmias
Centre Hospitalier de Luxembourg - CHL
Gloria MONTANES
gloria.montanes@lih.lu
+352 26 970 757
for articles/videos/studies
This page provides the list of clinical studies currently registered in the LuxCLIN platform in the different therapeutic areas. By clicking on each study title, more information is displayed concerning the study objective and the participation conditions.